CELL MECHANOBIOLOGY
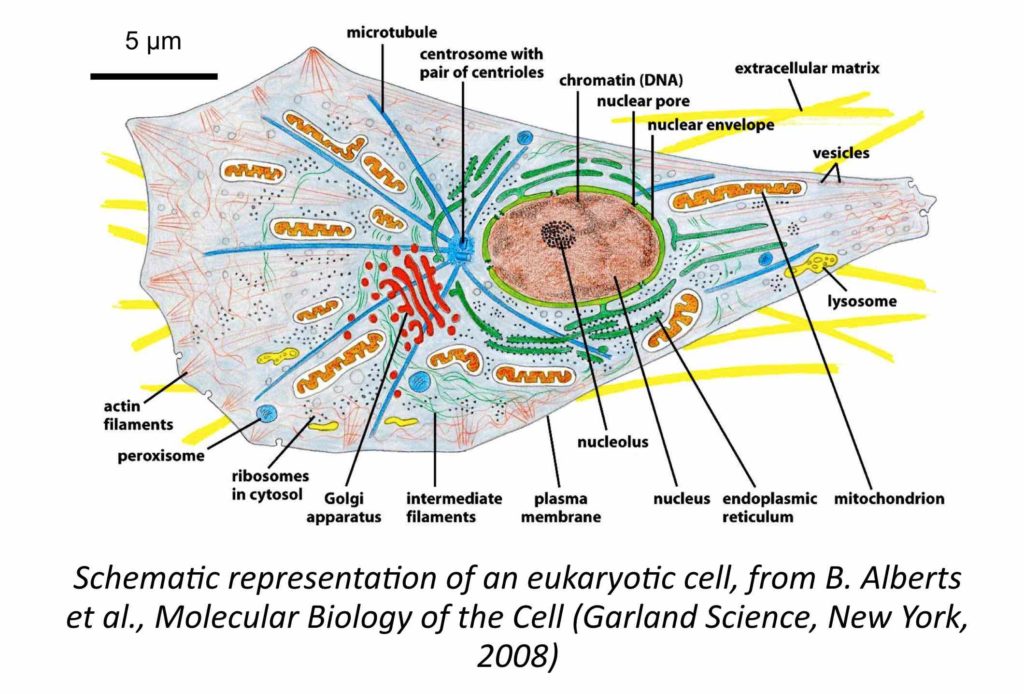
With living eukaryotic cells, we address the question of the viscoelastic nature of the cytoplasm, an issue that is relevant to understand how cells adapt to their environment and how forces are transmitted at the scale of individual cells. Recent studies have suggested that the cytoplasm biomechanical response is analogous to that of a weak elastic solid. Other work has led to the conclusion that the interior of living cells is equivalent to a viscous Newton fluid.
To test these hypotheses, cytoplasm viscosity measurements were performed on different cells lines including NIH/3T3 mouse fibroblsats, HeLa cervical cancer cells and A549 lung carcinoma epithelial cells using the magnetic wire microrheology techniques. When fibroblasts are exposed to the wires in Petri dishes, they sediment on the cell layer and over a few hours are spontaneously internalized. Once inside, the wire is subjected to a rotational magnetic field at an angular frequency between 10-2 and 10 rads-1, and their movement is monitored using time-lapse optical microscopy. The cytoplams viscosity is determined from the critical frequency which delimits the transition from a synchronous to an asynchronous motion. The determination of the static shear viscosity (10 – 100 Pa s) and elastic modulus (5 – 20 Pa) confirms the viscoelastic character of the cytoplasm.
With this study, we could demonstrate that the magnetic wire-based microrheology is a powerful technique able to determine the rheological nature of viscoelastic materials, and provide quantitative rheological parameters.
REFERENCES
C. Bostoen*and J.-F. Berret*
A mathematical finance approach to the stochastic and intermittent viscosity fluctuations in living cells
Soft Matter 16, 5959 – 5969 (2020)
https://doi.org/10.1039/C9SM02534K
J.-F. Berret*
Local viscoelasticity of living cells measured by rotational magnetic spectroscopy
Nature Communications 7, 10134 (2016)
https://doi.org/10.1038/ncomms10134